As the central organizing body for research at Humber River Health (HRH), the Research Department is dedicated to providing responsive, effective, and complete life-cycle support for the conduct of clinical trials and research that will have significant clinical and scientific impact for our patients’ healthcare.
The Research Department acts as a liaison between Physician Investigators and other groups, such as sponsoring agencies, Clinical Research Organizations (CROs), collaborating institutions and various offices, both on- and off-site. We strive to balance a high level of support to aid Physician Investigators and clinical researchers by ensuring appropriate stewardship, regulatory and financial compliance for all types of research initiatives.
The Research Department provides the following support:
Clinical Trials Management: We are a team of clinical research administrators, study coordinators and research assistants who provide dedicated administrative and research support services specifically adapted to address the unique requirements of each Physician Investigator, researcher, or clinical research site.
For Physician Investigators with limited administrative support, our study coordinators are available to assist with end-to-end clinical trial administration and protocol implementation.
For clinical research sites with an existing support structure already in place, our staff are flexible and available to provide additional administrative and research support services.
Our primary goal is to help manage all research activities at HRH such that a Physician Investigator or any researcher can focus their efforts solely on research objectives and patient care.
Research Pre- and Post- Awards Administration: We offer a full-service approach to support Physician Investigators and researchers with preparing research proposals, funding opportunity searches, study budget preparations and study budget justification. We provide support for establishing budget projections and allocations. We also help the research sites with the administration of pre-study spending and accounts, budget revisions or changes of scope. We serve as the financial planner throughout the study’s life cycle.
Research Contracts and Agreements: We review, negotiate and accept research agreements on behalf of Humber River Health. We provide assistance to the Physician Investigator in developing subcontracts, industry or non-industry clinical trial agreements, and research consulting agreements.
Research Education and Training: We provide assistance on questions related to local, federal, departmental, or standard operational policies associated with research. We are committed to establishing quality training standards and compliance to standard operating procedures at HRH. We are the Physician Investigator’s resource for any clinical research training requirements, including access to training in Health Canada Food and Drug Regulations (FDR), the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2), and The International Conference on Harmonization: Good Clinical Practice (ICH:E6).
Research Development and Compliance: We help researchers be up-to-date with all requirements for developing, collaborating, and conducting a research study. We help ensure that research best practices, compliance and data integrity are maintained for any study undertaken by our Physician Investigators and researchers. We understand that the goal of research involvement by HRH Physician Investigators is ultimately the improvement of healthcare, and we strive to ensure the welfare of all of our research participants, as they are HRH patients first and foremost.
You are encouraged to contact the Research Department for further information regarding research initiatives, collaborations, training and support, or to become involved in our growing research team at Humber River Health.
Research Department
Humber River Health
Clinical Research Office
416-242-1000 ext.81263
research@hrh.ca
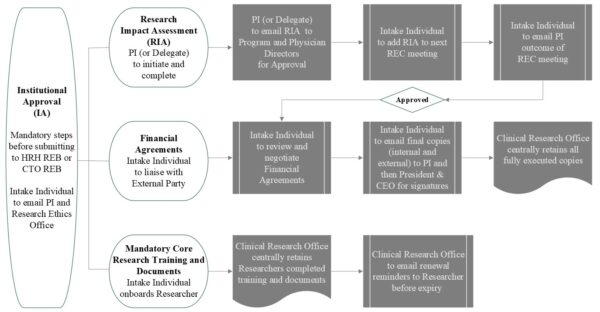
Institutional Approval (IA) is required for any research that uses resources, data, programs and/or services of Humber River Health (HRH) or its patients, clients, residents or staff, and is inclusive of considerations for organizational risks. IA is required for any research that will be conducted at or under the auspices of HRH. The Principal Investigator (PI) must obtain IA before submitting to the HRH REB (or CTO Qualified board).
Institutional Approval (IA) includes:
- Research Impact Assessment (RIA) Form submission and final approval by Research Executive Committee
- Financial Agreements, if there is funding with an external party
- Mandatory Core Research Training & Documents for the PI and research team (see Education & Training tab)
To speak to our Research Manager (and Intake Individual), you may call 416-242-1000 ext. 81263 or email research@hrh.ca.
Submission Process and Meeting Dates
Forms and Guidelines
Humber River Health understands that, in the field of medicine, tomorrow’s knowledge is the result of today’s research. As an organization, we are pleased to offer support to those wishing to pursue research activities in partnership with our hospital.
For single-centered research projects, please contact our Humber River Health REB.
For multi-centered research projects, please contact the CTO Institutional Representative.
Research training is required for anyone who conducts or is involved in human research activities at Humber River Health. To promote the highest quality, ethical and compliant conduct of research, completion of the following training courses is required for all researchers (depending on research type) and research team prior to conducting human participant research.
Clinical Research Office tracks completion of the applicable training courses for each PI. Institutional Approval (IA) to proceed with new research project(s) will be tied to course completion. It is the responsibility of the PI to ensure research team listed on the REB submission and/or Task Delegation Log have completed all applicable training prior to their study involvement.
Any questions may be directed to the Clinical Research Office at research@hrh.ca
Mandatory Core Research Training
Dr. Abhimanyu Sud - Research Chair in Primary Care and Population Health Systems
Nadine Akbar - Research Chair in Community Connection
Pete Wegier - Research Chair in Optimizing Care Through Technology




